BEHIND KANJINTI®
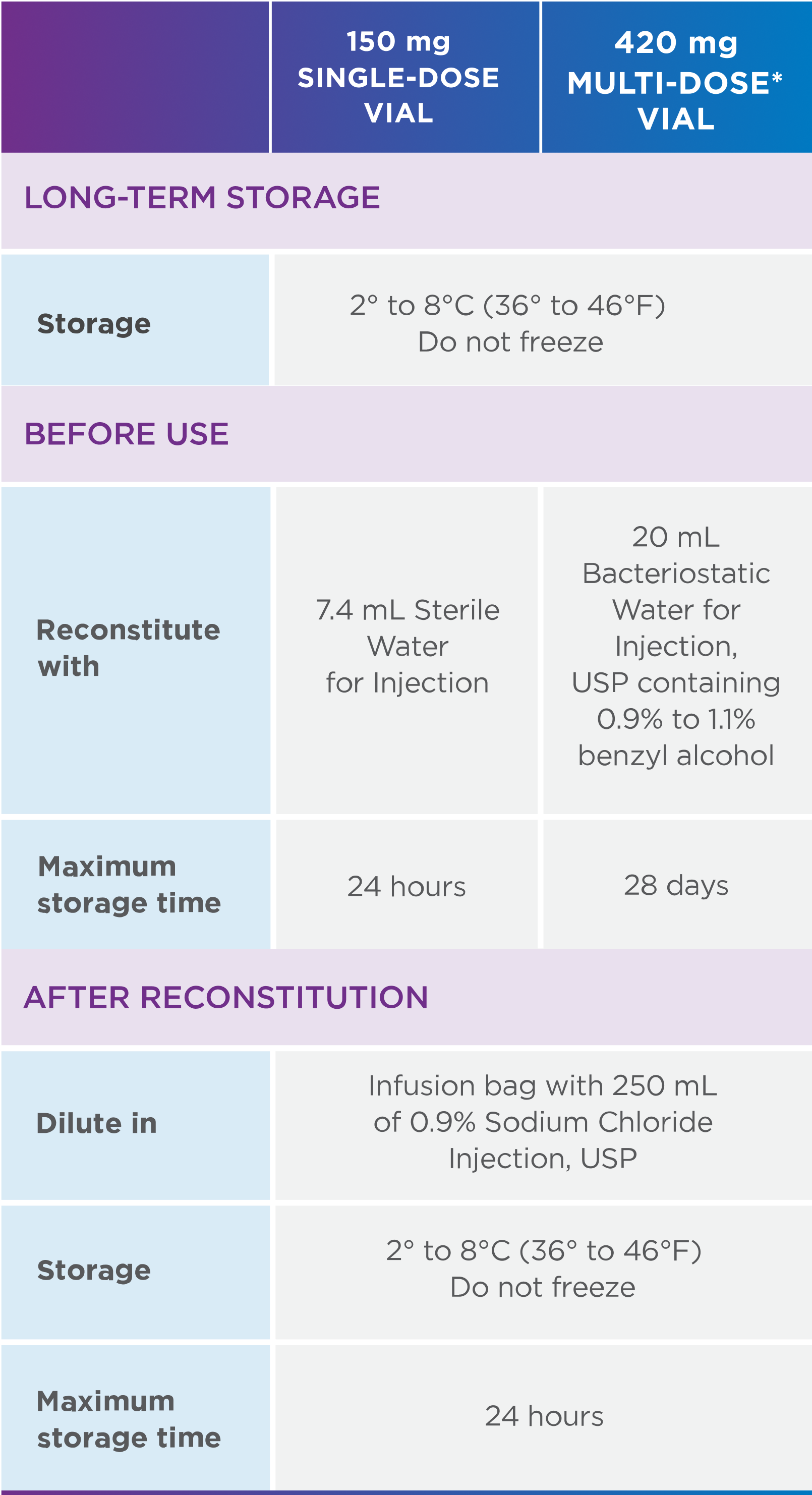
IDENTICAL STORAGE AND HANDLING1
†As of [June 30, 2025]. Number of non-oncology patients calculated from duration of therapy and adherence data from IQVIA Analytics Link and IQVIA Patient Link, respectively. Number of oncology patients determined using claims data (US) and physician surveys (ex-US). Patient count estimates may not accurately reflect the actual number of patients treated with Amgen biosimilars if adherence or length of treatment in real world differs from the assumptions used.
‡EU-sourced Herceptin® was used in the LILAC study. The KANJINTI® clinical pharmacokinetic (PK) study demonstrated equivalence between EU- and US-sourced Herceptin® and KANJINTI®.