Behind KANJINTI®
ANSWERING THE
GROWING
NEED FOR
BIOSIMILARS
Amgen is a leader in biologics with a growing portfolio of biosimilars.
HERITAGE
The healthcare community has been able to trust us for decades



AT AMGEN, WE’RE COMMITTED TO DELIVERING QUALITY PRODUCTS TO EVERY PATIENT, EVERY TIME
EXPERTISE
Real-world experience and expertise paved the way for our leadership in biosimilars


*Available biosimilars across all global markets.
†As of [June 30, 2025]. Number of non-oncology patients calculated from duration of therapy and adherence data from IQVIA Analytics Link and IQVIA Patient Link, respectively. Number of oncology patients determined using claims data (US) and physician surveys (ex-US). Patient count estimates may not accurately reflect the actual number of patients treated with Amgen biosimilars if adherence or length of treatment in real world differs from the assumptions used.
Commitment
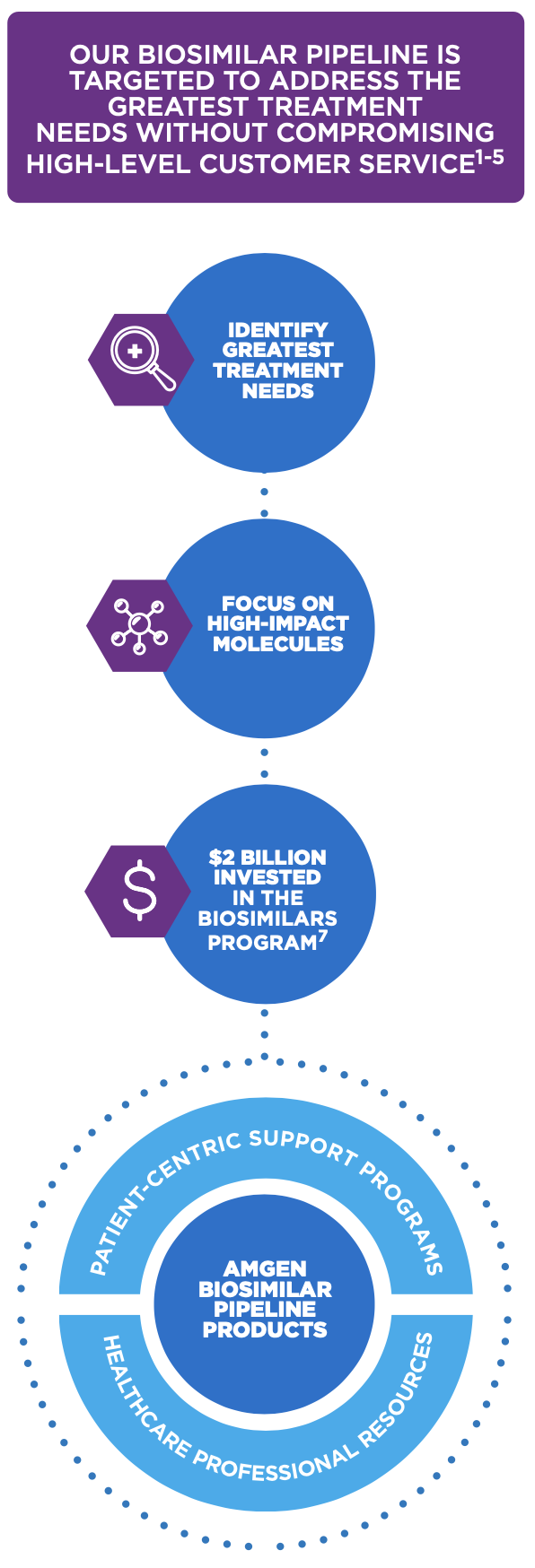
WE ARE A LONG-STANDING BIOTECHNOLOGY LEADER COMMITTED TO
DEVELOPING THE BIOSIMILAR MARKETPLACE
Invested in the development of biosimilars and novel therapeutics

Support
WE ARE COMMITTED TO PROVIDING SOLUTION-ORIENTED RESOURCES THAT CAN HELP PROVIDE POSITIVE BIOSIMILAR EXPERIENCES


Learn more about Amgen’s commitment to biosimilars.
Learn how Amgen uses the same support programs
for KANJINTI® as we do for our innovative treatments.
 support
supportTo stay up-to-date on Amgen’s advances in oncology, visit AmgenOncology.com.

